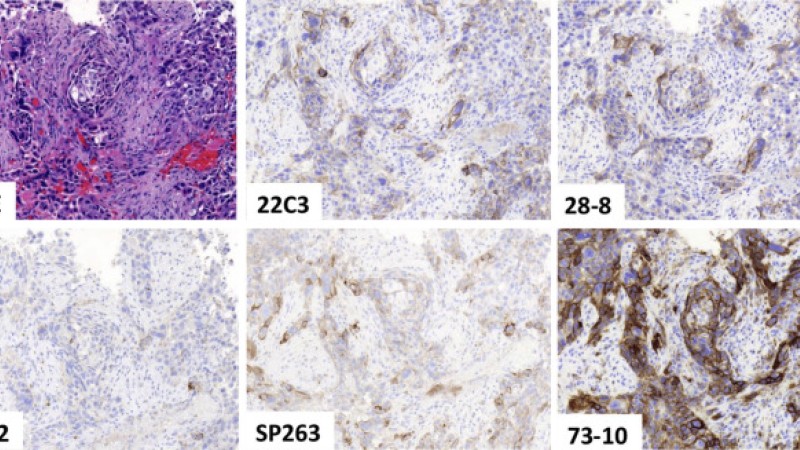
Blueprint Phase 2 Project
The Blueprint (BP) Programmed Death Ligand 1 (PD-L1) Immunohistochemistry Comparability Project is a pivotal academic/professional society and industrial collaboration to assess the feasibility of harmonizing the clinical use of five independently developed commercial PD-L1 immunohistochemistry assays. The goal of BP phase 2 (BP2) was to validate the results obtained in BP phase 1 by using real-world clinical lung cancer samples.
Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors
The "Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors" continues to set evidence-based standards for clinical molecular testing of non-small cell lung cancers (NSCLC) that effectively guides targeted therapy and treatment.